








Developing solvent extraction and direct electrodeposition processes for the volume reduction of secondary wastes is an important task.
Direct electrodeposition by employing ionic liquids (ILs) in a solvent based on the aqueous solution-IL distribution equilibrium is important for the extraction process.
Thus far, ILs have been evaluated for possible application in various stages of the recovery of metallic species.
Recently, we demonstrated the recovery of platinum group metals from a loaded organic phase using phosphonium-based ILs by solvent extraction and direct electrodeposition.
This study is working as part of Grant-in-Aid for Scientific Research (B)(23H02002).
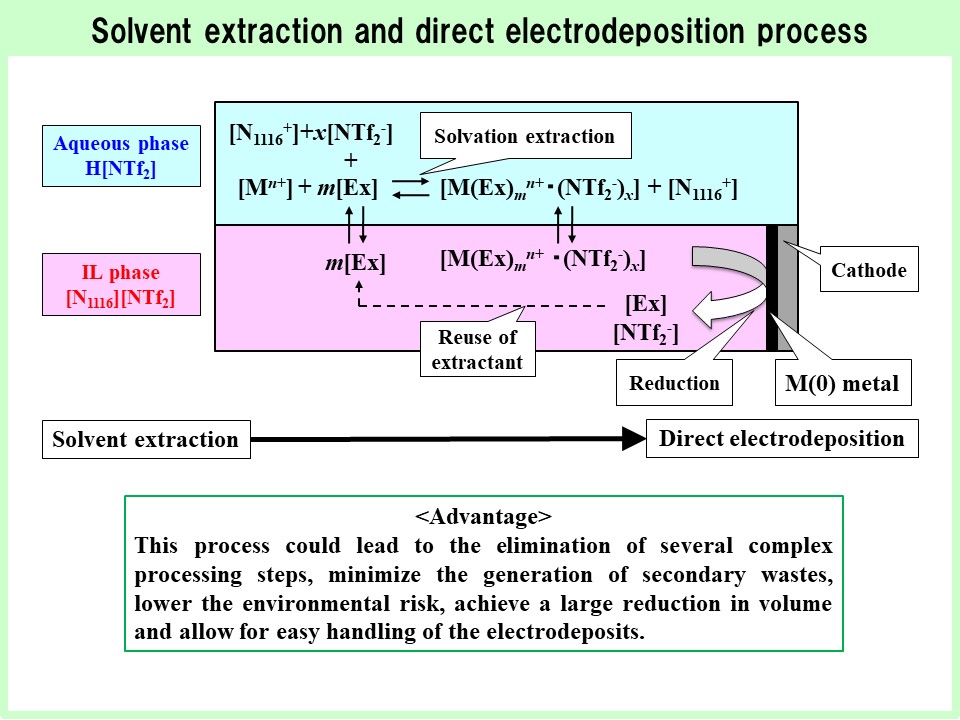
※Research results
・M. Matsumiya, R. Kinoshita, Y. Sasaki, J. Electrochem. Soc., 169 (2022) 082513.
・R. Kinoshita, M. Matsumiya, Y. Sasaki, Solvent Extr. Ion Exch.,40(6) (2022) 606-619.
・M. Matsumiya, Y. Tsuchida, R. Kinoshita, Y. Sasaki, J. Electrochem. Soc., 168(7) (2022) 076508.
・M. Matsumiya, R. Kinoshita, Y. Tsuchida, Y. Sasaki, J. Electrochem. Soc., 168(5) (2022) 056501.
・M. Matsumiya, Y. Song, Y. Tsuchida, Y. Sasaki, Sep. Purif. Technol., 234 (2020) 115841.
・M. Matsumiya, Y. Song, Y. Tsuchida, H. Ota, K. Tsunashima, Sep. Purif. Technol., 214 (2019) 162-167.
・Y. Song, Y. Tsuchida, M. Matsumiya, K. Tsunashima, Hydrometallurgy, 181 (2018) 164-168.
・M. Matsumiya, M. Sumi, Y. Uchino, I. Yanagi, Sep. Purif. Technol., 201 (2018) 25-29.
・M. Matsumiya, T. Yamada, Y. Kikuchi, S. Kawakami, Solvent Extr. Ion Exch., 34(6) (2016) 522-534.
・M. Matsumiya, T. Yamada, S. Murakami, Y. Kohno, K. Tsunashima, Solvent Extr. Ion Exch., 34(5) (2016) 454-468.
・M. Matsumiya, Y. Kikuchi, T. Yamada, S. Kawakami, Sep. Purif. Technol., 130 (2014) 91-101.
※Research grant
・Grant-in-Aid for Scientific Research (B) (23H02002)
・JST Adaptable and Science Technology transfer Program (A-STEP) (MP27115663745)
・Grant-in-Aid for Exploratory Research (15K14193)
・Sasakawa Scientific Research Grant, Nanae Tsuda (25-313)
・JST Adaptable and Science Technology transfer Program (A-STEP) (AS242Z02857M)
・JST Adaptable and Science Technology transfer Program (A-STEP) (AS232Z01827C)
We are focused on “urban mining”, which means that there are concentrated metal resources included in the
manufactured products at the end of their lives. The valuable metals in high-tech products have higher quality than those in the mines, so that the recycling of these rare metals is relatively easier than
recovering the rare metals from the mine. In addition, it is essential for our country to efficiently recover these metal resources from urban mining. Thus effective recovery from the wastes of high-tech
products leads to not only less environmental risks but also restraint of the overall wastes. In the case of neodymium-type magnets, most of the material wastes including a part of scrap
metal have remained in the manufacturing process so far. As for urban mining, a large amount of rare-earth metal was hardly used for recycling. Therefore we have studied the way of the recycling
neodymium and dysprosium metals from these products, especially VCM(Voice Coil Motor) in HDD. It is also important for our country to establish an original energy-saving recycling process of rare-earth metals.
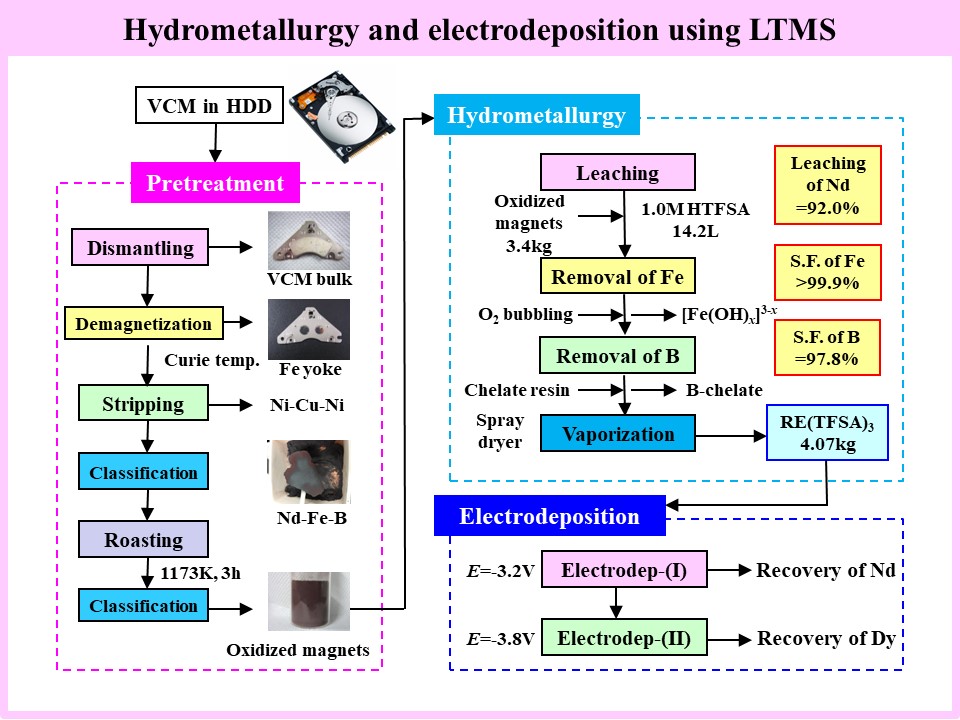
※Research results
・D. Nomizu, Y. Sasaki, M. Kaneko, M. Matsumiya, S. Katsuta, J. Radioanal. Nucl. Chem., 331 (2022) 1483-1493.
・M. Matsumiya, D. Nomizu, Y. Tsuchida, Y. Sasaki, J. Electrochem. Soc., 168(5) (2022) 056502.
・Y. Sasaki, M. Matsumiya, Y. Tsuchida, Anal. Sci., 36(11) (2020) 1303-1309.
・Y. Sasaki, Y. Ban, K. Morita, M. Matsumiya, R. Ono, H. Shiroishi, SERDJ, 27(1) (2020) 63-67.
・S. Murakami, M. Matsumiya, Y. Sasaki, S. Suzuki, S. Hisamatsu, K. Takao, Solvent Extr. Ion Exch., 35(4) (2017) 233-250.
・M. Matsumiya, H. Ota, K. Kuribara, K. Tsunashima, J. Electrochem. Soc., 164(8) (2017) H5230-H5235.
・H. Ota, M. Matsumiya, T. Yamada, T. Fujita, S. Kawakami, Sep. Purif. Technol., 170 (2016) 417-426.
・S. Murakami, M. Matsumiya, T. Yamada, K. Tsunashima, Solvent Extr. Ion Exch., 34(2) (2016) 172-187.
・M. Matsumiya, M. Ishii, R. Kazama, S. Kawakami, Electrochim. Acta, 146 (2014) 371-377.
・K. Ishioka, M. Matsumiya, M. Ishii, S. Kawakami, Hydrometallurgy, 144-145 (2014) 186-194.
・R. Kazama, M. Matsumiya, N. Tsuda, K. Tsunashima, Electrochim. Acta, 113 (2013) 269-279.
・A. Kurachi, M. Matsumiya, K. Tsunashima, S. Kodama, J. App. Electrochem., 42(11) (2012) 961-968.
・H. Kondo, M. Matsumiya, K. Tsunashima, S. Kodama, Electrochim. Acta., 66(1) (2012) 313-319.
※Research grant
・JST Adaptable and Science Technology transfer Program (A-STEP) (JPMJTM22C1)
・JST Adaptable and Science Technology transfer Program (A-STEP) (JPMJTM20D6)
・Sasakawa Scientific Research Grant, Daiki Nomizu (2021-3040)
・Grant-in-Aid for Scientific Research (B) (18H03404)
・JST Adaptable and Science Technology transfer Program (A-STEP) (VP29117940881)
・Grant-in-Aid for Scientific Research (B) (15H02848)
・Sasakawa Scientific Research Grant, Naoko Sasaya (27-334)
・JST Adaptable and Science Technology transfer Program (A-STEP) (AS2621394M)
・Grant-in-Aid for Young Scientists (A) (24686101)
The ionic liquids media have some unique properties such as low vapor pressure, low environmental-diffusivity. Such characteristics are hopeful to apply the chemical reaction media and the electrochemical devise. The motion of ionic species is dominated by the interaction between cation and anion. In addition, it is necessary to analyze both microscopic and macroscopic properties in ionic liquids in order to reveal these intercations.Solvation and diffusive properties are important factor for electrodeposition process in ionic liquids media. In our laboratory, the solvation structure is analyzed by Raman spectroscopy with DFT calculations. Moreover, the bulk properties such as self-diffusion coefficient, viscosity, ionic conductivity in ionic liquids media have investigated by MD simulation.
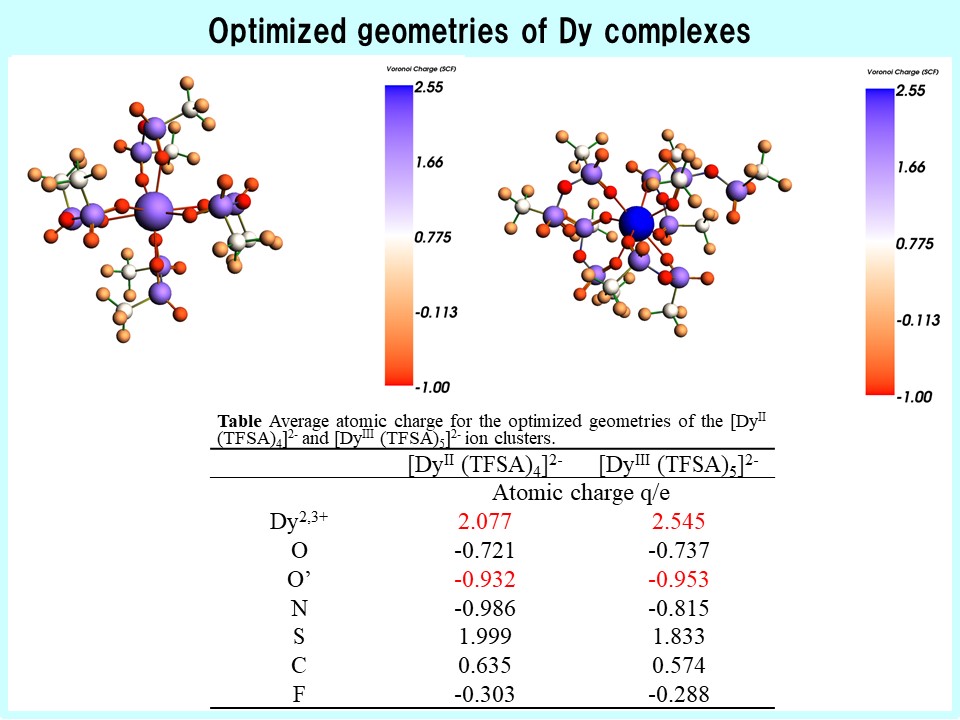
※Research results
※Research grant
・D. Nomizu, Y. Tsuchida, M. Matsumiya, K. Tsunashima, J. Mol. Liq., 318 (2020) 114008.
・Y. Tsuchida, M. Matsumiya, K. Tsunashima, J. Mol. Liq., 269 (2018) 8-13.
・M. Matsumiya, R. Kazama, K. Tsunashima, J. Appl. Sol. Chem. Model., 5(4) (2016) 157-167.
・M. Matsumiya, R. Kazama, K. Tsunashima, J. Mol. Liq., 215 (2015) 308-315.
・M. Matsumiya, K. Hata, K. Tsunashima, J. Mol. Liq., 203 (2015) 125-130.
・M. Matsumiya, Y. Kamo, K. Hata, K. Tsunashima, J. Mol. Struct., 1048 (2013) 59-63.
・Sasakawa Scientific Research Grant, Ryoma Kinoshita (2022-2027)
・Sasakawa Scientific Research Grant, Ryo Kazama (26-216)